Martin Tabakov, Ph.D. Eng.
The Biomedical Informatics team of the Department of Computational Intelligence conducts research on the introduction of faster and cheaper method of diagnosing the degree of HER-2/neu protein overexpression, at the stage of immunohistochemistry (IHC) processing, through advanced analysis of histopathological images taken from medical microscopes.
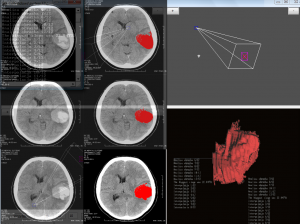
The Biomedical Informatics team of the Department of Computational Intelligence conducts research on the development of advanced algorithms for 3D reconstruction of selected pathological structures, such as hematoma, brain tumors etc., which are extracted from Computed Tomography or Magnetic Resonance digital images. The study also includes the introduction of automatic segmentation algorithms of target pathological areas.
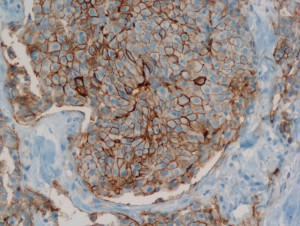
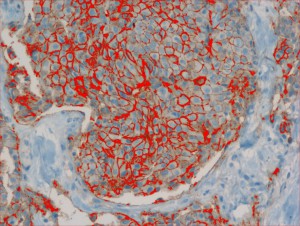
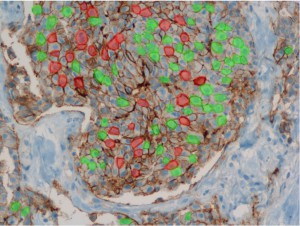
Analysis of histopathological images
 |
 |
 |
The human epidermal growth factor receptor 2 (HER-2/neu) is a biomarker, recognized as a valuable prognostic and predictive factor for breast cancer. In approximately 20% of primary breast cancers, the HER2/neu protein is over-expressed. The effect of this over-expression is an increase in receptor mediated intracellular signalling, directing the cancer cells into fast proliferation, which results in an aggressive form of breast cancer. The activity of this receptor may be blocked via utilization of monoclonal antibodies, which clinical usefulness was confirmed in clinical trials. One of such agents is the widely utilized trastuzumab (trade name: Herceptin).
Only patients whose breast cancers are characterized by amplification of HER-2/neu may benefit from such specific agents, therefore strict patients qualification is immensely important. Potential responders to trastuzumab treatment are currently identified in a two-step procedure. The first step is based on examination of membranous cell staining in tumour cells in immunohistochemical sections of breast cancer. The results are categorized according to a semi-quantitative four–grade scale: 0 (no staining), 1+ (incomplete, weak membrane staining regardless of the proportion of tumour cells stained), 2+ (nonuniform complete membrane staining or staining with obvious circumferential distribution in at least 10% of the tumour cells, or intense, complete membrane staining ≤30% of the invasive tumor cells), 3+ (intense membrane staining in >30% of the invasive tumour cells). Cases scored 3+ utilizing this method are regarded as HER2/neu over-expressing cases and qualified for the trastuzumab based therapy. Cases classified as 0 and 1+ are not. Sections scored as 2+ are regarded as unequivocal and require additional testing using a costly fluorescent in situ hybridization technique (FISH) for final determination of HER-2/neu amplification status.
http://www.tvn24.pl/wroclaw,44/system-komputerowy-pomoze-onkologom-wykryc-raka,509028.html
Computed Tomography and Magnetic Resonance digital images
The combination of computerized imaging and medicine has experienced an explosive growth in recent years due to several imaging techniques, such as X-ray, computed tomography (CT), magnetic resonance (MR) imaging, positron emission tomography (PET), single positron emission tomography (SPECT), and ultrasound. The digital techniques and the computers processing power in combination with these imaging modalities have helped humans to better understand the complex human anatomy and it behaviour to a certain extend. Computer power and medical scanner data alone are not enough there is still the need of the art to extract the necessary boundaries, surfaces, and segmented volumes of different anatomical structures in the spatial and temporal domains. This process of organ extraction is called segmentation. Image segmentation is essentially a process of pixel classification, wherein the image pixels are segmented into subsets by assigning the individual pixels to classes. The main objective of a medical image segmentation is to extract and characterise anatomical and/or pathological structures with respect to some input features and/or expert knowledge. These segmented organs and their boundaries are very critical in the quantification process for physicians and medical surgeons in any branch of medicine which deals with imaging.
The next step, after image segmentation, is the process of recognition and/or the 3D reconstruction of the segmented structures. The recognition process gives significant information about the anatomy specificity, being under investigation, which may be used for example to plan corresponding surgery procedures. The 3D reconstruction process is critical for spatial analysis, as it brings important information on the location of the pathological areas and gives the opportunity to make an exact visualization.